JUUL LABS PRESENTS NEW DATA ON SECONDHAND VAPOR, ROLE OF FLAVORED PRODUCTS IN SWITCH RATES AND PUFF TOPOGRAPHY AT THE 6TH GLOBAL FORUM ON NICOTINE
Research & Analysis
June 14, 2019
This week, we presented new data from three studies that shed light on important environmental, behavioral and product-use factors associated with the use of vapor products, including JUUL. The results were presented at the 6th Global Forum on Nicotine in Warsaw, Poland.
Cigarette smoking remains the number one cause of preventable death worldwide, accounting for more than 8 million deaths each year from both direct use and indirect exposure to combustible smoke. It is critical that we find alternative options to combustible cigarettes for the world’s 1.1 billion adult smokers1and those around them, which is why we are pleased to have the opportunity to share our research at this year’s Forum.
ENVIRONMENTAL EXPOSURE ASSOCIATED WITH THE USE OF VAPOR PRODUCTS
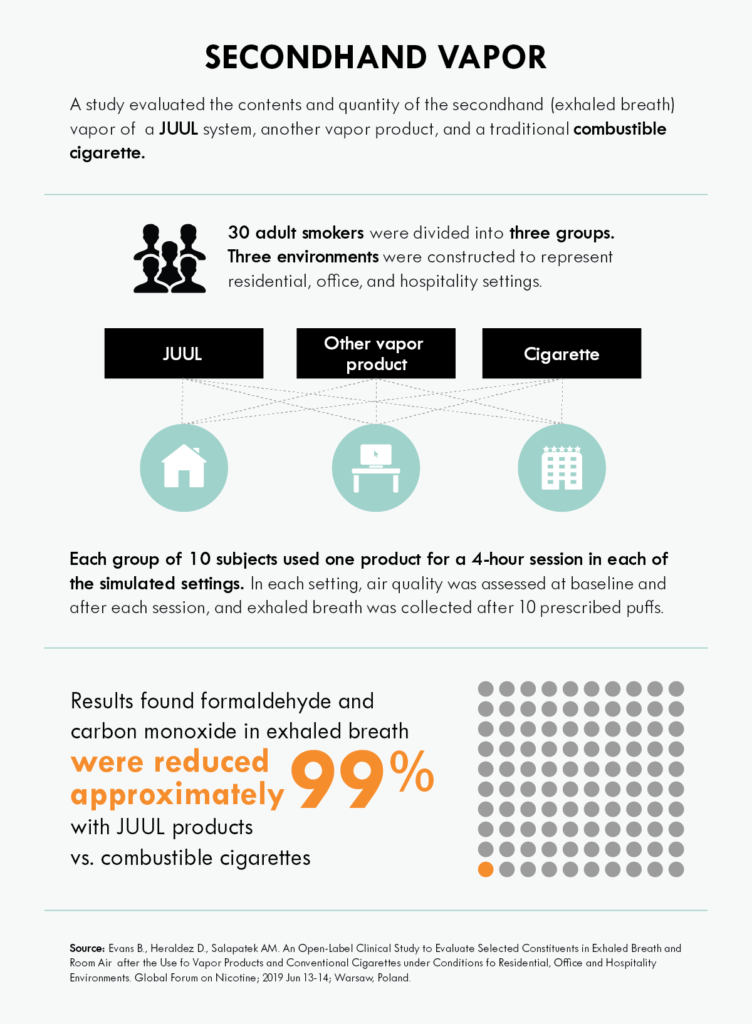
Results from a clinical study of 30 adult smokers found an approximately 99% reduction of formaldehyde and carbon monoxide particles in secondhand (exhaled breath) vapor associated with the use of the JUUL system compared to the use of combustible cigarettes. The aggregate measurements of formaldehyde and carbon monoxide particles were not statistically different from the background levels measured without product use.
In air quality analyses, concentrations of respirable particles were elevated in every environment and product evaluated. However, the average rise in airborne particles was lower with the JUUL system versus the other vapor product and combustible cigarettes. Room air nicotine levels were 89%–95% lower following ad libitum vapor product use (using the product at their discretion) versus cigarettes. The study authors note the presence and variability of background environmental source chemicals as a factor in the analysis.
These findings align with the current scientific understanding of the role alternative nicotine delivery systems can play for adult smokers and the corresponding environmental impact for those around them.
NON-TOBACCO-FLAVORED JUUL PRODUCTS ASSOCIATED WITH HIGHER LIKELIHOOD OF SWITCHING FROM FROM COMBUSTIBLE CIGARETTES
Results from another study demonstrate adult smokers who primarily used non-tobacco-flavored JUUL products were more likely than those who primarily used tobacco-flavored JUUL products to have successfully switched from smoking combustible cigarettes over a 6-month period.
The study followed more than 37,000 US adult smokers who had recently purchased JUUL products at retail or on the company’s e-commerce platform. Participants completed online surveys over 6 months.
Researchers analyzed data from a subset of 21,332 smokers aged 21+ years who had purchased a JUUL starter kit and completed at least two follow-up assessments. Study participants completed baseline and 30-day, 60-day, 90-day and 180-day follow-up assessments via online surveys. The assessments asked study participants about JUUL flavor use and switching—defined as no smoking, not a single puff— for the previous 30 days prior to the assessment. JUUL Labs commissioned and funded the study, Centre for Substance Use Research (CSUR) independently designed and administered the survey underlying this data analysis.
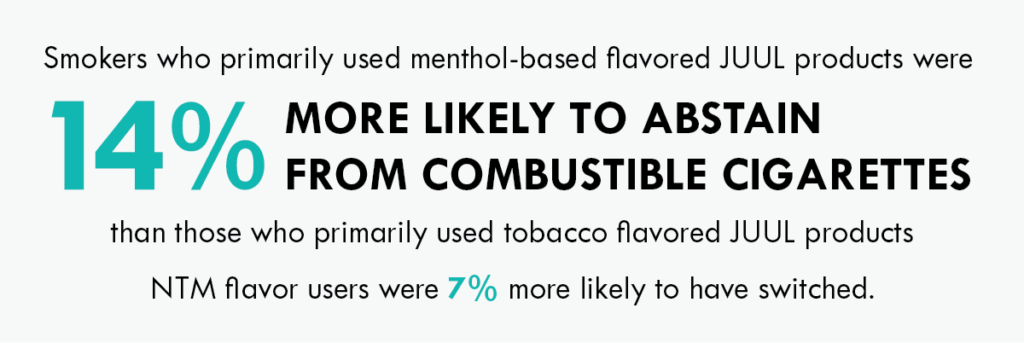
Across 30-, 60- and 90-day assessments, use of menthol-based (i.e., Menthol and Mint) and non-tobacco/menthol-based (NTM) flavored JUUL products, compared with the use of tobacco flavored JUUL products, were positively associated with past 30-day smoking abstinence at the 60-, 90- and 180-day assessments. Adult smokers who primarily used menthol-based and NTM flavors were 14% and 7% more likely, respectively, to have switched than those primarily using tobacco flavors across the 6-month study period.
There has been an active discussion about the role of flavors in supporting adult smokers who want to switch from combustible cigarettes and, at JUUL Labs, we are committed to driving research to inform these discussions. These results add to a growing body of evidence that suggest that the use of vapor products in non-tobacco flavors may potentially help adult smokers switch from combustible cigarettes compared to tobacco flavors.
ADDITIONAL INSIGHT INTO INHALATION PATTERNS (“PUFF TOPOGRAPHY”) IN ADULT SMOKERS
A third study sought to measure real-life puffing behaviors of 30 adult smokers when using JUUL Virginia Tobacco (5% nicotine) over the course of 15 days. On Day 1 and Day 15 of the study, subjects were asked to use the product as they normally would. Researchers then assessed a number of parameters including puff duration, puff volume, flow rate and time between puffs.
Investigators observed a correlation between initial enjoyment of the product and increases in ongoing use parameters, including puff count, puff volume and puff duration. In contrast, adult smokers who reported lower enjoyment tended to diminish use over time and to take shorter puffs over time.
Understanding how adult smokers interact with JUUL products is important because it helps ensure our other clinical and preclinical test conditions, including mechanized puff machines, are correctly calibrated to mimic real-world use of the product among adult smokers, including puff duration and volume. Puff topography studies also provide valuable insight into how the initial use and enjoyment of the product can influence future switching behavior.
Data were collected at Rose Research Center in Raleigh, North Carolina, using the CReSS Pocket, a version of the widely-used Clinical Research Support System for Laboratories reference system. The Center specializes in tobacco dependence research, including research on adult smokers, addiction, smoking cessation, tobacco harm reduction and the use of other tobacco products.
Ongoing inquiry into puff topography and overall user patterns will be important to further the scientific understanding of the potential impact of vapor products as a viable alternative to combustible cigarettes.
For more information on all of the data presented, please visit jliscience.com.
JUUL Labs is committed to both conducting and supporting durably designed, painstakingly executed preclinical, clinical, and behavioral research examining the potential public health impact of our products. We are excited to have been a part of one of the largest international conferences on nicotine research and sciences, and look forward to presenting more data going forward on our mission to eliminate combustible cigarettes among adult smokers.
Other Posts
October 25, 2024
ROBYN GOUGELET DELIVERS REMARKS DURING A PUBLIC MEETING HOSTED BY FDA AND NIH
On October 21, Juul Labs’ Vice President of U.S. Regulatory Affairs, Robyn Gougelet, delivered remarks during a joint public meeting hosted by the U.S….
March 17, 2023
JUUL LABS PUBLISHES WHITE PAPER EXAMINING THE REAL-WORLD IMPACT OF ENDS PRODUCTS FOR ADULT SMOKERS
In a new white paper entitled “The Real-World Impact of ENDS for Adult Smokers: Tobacco Harm Reduction Through Real-World Data and Evidence,” Juul Labs has reviewed and compiled the latest science and evidence demonstrating the positive real-world impact of ENDS products for adult smokers.
March 17, 2022
PEER-REVIEWED STUDY FINDS BANNING VAPOR PRODUCTS MAY LEAD TO INCREASED CIGARETTE SALES
This research shows that policies that significantly restrict vapor products are likely deterring current adult smokers from switching and driving former adult smokers back to combustible cigarettes, which remain the leading cause of preventable death in the U.S. and worldwide.